What does “a warehouse licensed by the Food and Drug Authority” mean?
A warehouse licensed by the Saudi Food and Drug Authority (SFDA) is a facility that is subject to strict standards and regulations imposed by the authority to ensure the safety and quality of storage and handling of products such as medicines, medical devices, cosmetics, and food. These warehouses must obtain an official license from the authority after meeting all necessary conditions, including cleanliness, ventilation, and providing suitable storage conditions according to the nature of each product.
Development Partners Co. for Logistic Services is considered one of the leading companies that offer storage services in warehouses licensed by the Food and Drug Authority. It provides storage services and logistical support that comply with all conditions for storing medicines, medical devices, and cosmetics, in addition to product registration services and obtaining the necessary licenses for import and distribution.
What is meant by the term “third-party storage”?
“Third-party storage” refers to providing storage services for a third party. This means that the company or institution offering storage space allows customers to store their products without needing to own a private warehouse. This service makes the storage service provider responsible for maintaining the quality and safety of the stored products according to the required standards and conditions.
In 2019, the Saudi Food and Drug Authority (SFDA) launched the “third-party storage” service, which allows tenants to obtain an independent license to operate legally without the need for a municipal or civil defense license, and without the necessity of owning an entire warehouse. This service helps companies that do not require large warehouses to reduce costs.
To obtain a third-party storage license in a warehouse licensed by the SFDA, the following steps must be followed:
1. Ensure Compliance with Standards:
The warehouse must comply with the standards for safe storage of products such as food, medicines, cosmetics, or animal feed according to the requirements of the SFDA.
2. Submit an Application for a License:
An application for a third-party storage license is submitted through the electronic platform of the SFDA. The application requires documents such as the lease agreement, commercial register, and applicant authorization.
3. Inspection and Approval:
After submitting the application, the SFDA conducts an inspection of the warehouse to ensure it meets the required standards. If all standards are met, the license will be granted.
4. Pay Fees:
The fees vary based on the activity and the required space for obtaining or renewing the license.
Development Partners Co. for Logistic Services is licensed by the Saudi Food and Drug Authority to provide third-party storage services and offer support and consulting in this field. We are pleased to provide our services to you.
Are all of Development Partners’ warehouses licensed by the Saudi Food and Drug Authority (SFDA)?
Dear Partner,
Yes, all warehouses of Development Partners Co. for Logistic Services are fully licensed by the Saudi Food and Drug Authority (SFDA). We recognize the importance of storing regulated products such as medicines, cosmetics, medical devices, and food in licensed warehouses to ensure compliance with the highest safety and quality standards.
Storing products in an unlicensed warehouse can expose facilities and their owners to significant legal risks, including financial penalties, closure of the warehouse, or even criminal sanctions. Additionally, unlicensed warehouses may not comply with the required storage standards, which can affect the quality and safety of the products.
At Development Partners Co., we are committed to providing a comprehensive and certified storage environment to ensure that products reach consumers with high quality and full effectiveness. Adhering to licenses and stringent standards ensures the preservation of the facility’s reputation and the trust of business partners, as well as facilitating the acquisition of appropriate insurance for stored products.
Development Partners Co. for Logistic Services pays attention to all details and strives to store your products in warehouses licensed by the Food and Drug Authority, ensuring they reach the consumer in their high quality.
Do you store all products?
We specialize in storing products regulated by the Food and Drug Authority only, which include:
• Human Medicines
• Medical Devices
• Cosmetics
We are committed to the highest safety and quality standards to ensure the effectiveness and safety of these products throughout the storage period.
Is it possible to store goods without obtaining a warehouse license (third-party storage)?
No, in order for us to receive your goods in our warehouses, a warehouse license (third-party storage) must be obtained in advance by the partner. This license ensures compliance with the standards and regulations set by the Food and Drug Authority to guarantee the safety and quality of the stored products.
What is the minimum duration of the contract?
According to the instructions of the Saudi Food and Drug Authority, the duration of the storage contract is only one year. After this period ends, the contract is renewed with a new storage agreement to ensure continued compliance with safety storage standards and regulations.
What is the minimum rental space available?
The minimum rental space depends on the type of products being stored:
• Medicines: 10 square meters.
• Medical devices and supplies: 2 square meters.
• Cosmetics: 10 square meters.
What is the standard unit of measurement for storage space that you use?
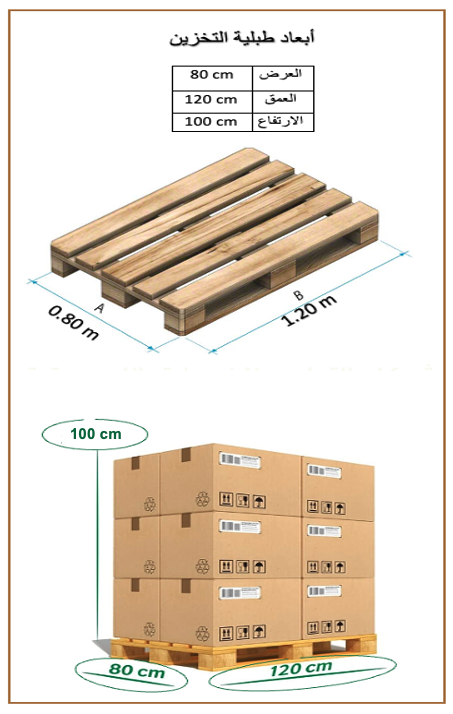
The unit of measurement for storage in our warehouses is the meter, taking into account the dimensions of one meter for storage in accordance with the conventional storage units.
Dimensions shown in the picture are per meter:
Do your warehouses have dedicated staff for loading and unloading goods?
Yes, we offer loading and unloading services (for large quantities) and provide a (forklift truck) for a nominal fee, which is paid with each operation.
How is the payment of lease contracts made?
Our lease contracts are annual, and the full contract amount is to be paid after signing via bank transfer to the company’s account only. We would like to emphasize that no cash amounts are accepted.
Do you have branches in areas outside Riyadh?
All our warehouses are currently located in Riyadh, and branches will soon be opened in other cities, and our partners will be notified accordingly.
Do you offer product registration and listing services with the Saudi Food and Drug Authority (SFDA)?
Yes, this service is provided after signing storage contracts, where we offer product registration and listing services with the Saudi Food and Drug Authority. We provide these services through licensed offices that have been contracted to offer the best rates for our partners. You can visit the following link for more details: Licensing Services page.
We need a full warehouse for rent. Do you offer this service?
Yes, at Development Partners Logistics Services, We provide full warehouse rental services dedicated to large companies that require independent and private storage spaces. We offer fully equipped and licensed warehouses from the Food and Drug Authority to meet all your storage needs according to the highest quality standards.
.
What is meant by renting storage space?
Renting storage space means that you are renting a portion of a warehouse that offers third-party storage services, where you can store your products such as medicines, medical devices, and cosmetics. These spaces are typically measured in cubic meters or by the number of pallets, providing a safe environment that complies with the necessary standards and conditions for product storage without the need to own a private warehouse.
In addition to storage, we offer additional services such as completing registration procedures with the Food and Drug Authority and consulting related to import and distribution. This type of service is suitable for companies that need flexible or temporary storage, or that wish to avoid the high costs associated with building and managing a private warehouse.
At Development Partners Co. for Logistic Services, we provide multiple options for storage spaces to meet the diverse needs of our partners. Renting storage space is an ideal choice for small and medium-sized businesses that require flexibility in inventory management and adaptation as their business grows without incurring high costs.
You can contact one of our staff members for more options.
What are the warehouses licensed by the Food and Drug Authority?
Licensed warehouses by the Saudi Food and Drug Authority (SFDA) are warehouses that are subject to strict standards and regulations imposed by the authority to ensure the safety and quality of storage and handling of products such as medicines, medical devices, cosmetics, and food. These warehouses must obtain an official license from the authority after meeting all necessary conditions such as cleanliness, ventilation, and providing appropriate storage conditions according to the nature of each product.
Development Partners Co. for Logistic Services is one of the leading companies that offer storage services in warehouses licensed by the Food and Drug Authority. It provides storage services and logistical support that comply with all the requirements for storing medicines, medical devices, and cosmetics, in addition to product registration services and obtaining the necessary licenses for import and distribution.
I only need a warehouse license and will not be storing with you. Do the prices differ?
Our services depend on reserving a specific space in our warehouses and obtaining storage and import licenses based on these spaces registered in the warehouse data with the Food and Drug Authority. These spaces are at the partner’s disposal throughout the contract period.
We recommend that all our partners keep and store products in designated locations approved by government agencies, to ensure that they reach the consumer in a fit-for-use condition.
What are the requirements for licensing a pharmaceuticals/medicines warehouse from the Food and Drug Authority?
There are requirements set by the Saudi Food and Drug Authority (SFDA) for obtaining a warehouse license for pharmaceuticals and herbal products, which include:
1. Commercial Register:
The commercial register must include the required activity according to the approved economic activities and must be issued from the same administrative region as the facility’s location.
2. Municipal and Civil Defense Licenses:
The warehouse must have a municipal license and a civil defense license to ensure compliance with regulatory requirements.
3. Contract with a Pest Control Company:
There must be a contract with a specialized pest control company to maintain a safe storage environment.
4. Qualified Saudi Technical Manager:
A qualified Saudi technical manager must be appointed according to the requirements of the SFDA.
5. Contract with a Company for Safe Disposal of Medical and Chemical Waste:
There must be a contract with a specialized company for the safe disposal of medical and chemical waste.
The application is submitted via the “Ghad” platform of the Food and Drug Authority, and the license is valid for five years, with a fee of 3,000 SAR.
If your facility wishes to rent a fully equipped warehouse that meets all requirements, you can check the complete warehouse rental section in our large enterprises services. You can also view the details of the licensing services we provide through the licensing services section.
What additional services do you offer?
Our services are not limited to warehouse rentals only. We have expanded our range of services to be comprehensive for our partners, in partnership with the best licensed offices from the Food and Drug Authority to provide the following services:
• Opening accounts with the Saudi Food and Drug Authority.
• Obtaining import licenses.
• Product registration and marketing permits.
• Registering facilities in the Medical Device National Registry (MDNR).
• Obtaining facility licenses (MDEL-I/D).
• Obtaining legal representative licenses for manufacturers (MDEL-AR).
• Obtaining quality system certification (ISO 13485).
• General consulting to ensure compliance with regulatory requirements.
When searching for a warehouse licensed by the SFDA for rent, what important notes should I focus on?
When you decide to search for a warehouse licensed by the SFDA for rent, you should consider several factors to ensure the selection of the appropriate warehouse for your needs. Here are some important points to consider:
- Location:
Choose a warehouse located in a strategic area suitable for your supply chain, close to major roads to reduce future transportation costs.
- Government Licenses:
Ensure that the warehouse you wish to rent storage space in has all the necessary licenses from the SFDA, the municipality, and civil defense, and that it complies with all regulations for the type of goods you intend to store (such as medicines, food, and medical devices).
- Essential Equipment:
Look for warehouses that are fully equipped and free from structural defects (such as water leaks and the type of paint used on walls and floors that can promote bacterial and fungal growth). Make sure they are equipped with temperature and humidity control systems if the products you are dealing with are sensitive to environmental conditions. Also, ensure there are security measures like surveillance cameras and alarm systems.
- Space and Future Expansion:
Choose a warehouse that provides enough space for your current and future needs. Consider the potential for expanding the space according to changes in your future business volume.
- Cost:
While it is natural for many to look for the lowest cost, always compare the rental costs of warehouses with the services provided. Consider all additional costs such as maintenance, security, insurance, storage quality, and the loading and unloading of goods.
- Contract Flexibility:
Examine the terms of the contract carefully, including the rental duration and renewal conditions. The contract should be flexible enough to adapt to market changes and business needs.
- Reviews and Ratings:
Look for reviews and evaluations of the companies providing the services. Previous tenants’ opinions and experiences can give you a good idea of the service level and potential issues.
- Site Visit:
Visit the selected warehouses for a personal assessment before making a final decision. Focus on how the space is organized, cleanliness, and the safety systems in place. Also, check for wide and suitable access and loading/unloading routes for the type of goods you plan to store.
Visiting the site can reveal details that may not be clear in photos or descriptions provided by the companies, such as the quality of maintenance and the effectiveness of daily operations. It also allows you to ask questions directly to the manager or staff and receive immediate answers that help evaluate whether the warehouse meets your expectations and requirements.
In summary, when choosing a warehouse licensed by the SFDA for rent, it is important to consider a variety of factors, from location and equipment to cost and contract. Ensure you conduct a comprehensive and thorough evaluation of the available options to guarantee the selection that best meets your needs and expectations.
At Development Partners Co. for Logistic Services, we always strive to achieve our partners’ visions and help them reach their business goals at the lowest cost and highest quality in our warehouses, which we continually work to make the best place for storing your products.
What are the requirements for registering medical devices with the Food and Drug Authority?
Registering a medical device with the Food and Drug Authority requires following several steps and procedures according to the guidelines set by the Saudi Food and Drug Authority (SFDA), which oversees this process to ensure the safety and effectiveness of medical devices. Here are some essential steps and main requirements:
1. Obtain a Facility License: A license for the import and distribution of medical devices must be obtained to ensure the legality of the import.
2. Classification of the Device to be Registered: The medical device is classified based on the level of risk associated with it, which is necessary before registering the product with the SFDA.
3. Documentation and Approvals: In some cases, you may need to provide approvals such as CE Marking or FDA approval based on the classification of the medical device.
4. Electronic Registration: The device must be registered through the electronic registration system for medical devices (MDNR) affiliated with the SFDA.
5. Submit Required Documents: Required documents include quality certificates, clinical studies, and technical reports that demonstrate the safety and effectiveness of the device.
6. Local Agent: A local agent must handle the registration process and communication with the SFDA to ensure a smooth operation.
7. Inspection and Evaluation: Medical devices may be subject to inspection and evaluation by the authority to ensure compliance with the required standards.
8. Periodic Follow-up: After obtaining a license from the SFDA, it is essential to maintain quality and safety standards and update documents as needed.
9. Registration Fees: The fees for licensing by the SFDA depend on the classification of the device and the type of registration:
• Class I = 15,000 SAR
• Class II A = 19,000 SAR
• Class II B = 21,000 SAR
• Class III and IV = 23,000 SAR
These are the basic steps for registering a medical device. We recommend contacting the SFDA directly or consulting our specialized advisors to assist in obtaining licenses and ensuring compliance with the SFDA licensing conditions.
What are the requirements for licensing a warehouse from the Food and Drug Authority (third-party storage)?
In 2019, the Saudi Food and Drug Authority (SFDA) launched the “Third-Party Storage” service, which allows tenants to obtain an independent license to operate legally without the need for a municipal or civil defense license, and without the necessity of owning an entire warehouse that may not fit the scale of their business activity.
According to the information provided by the authority on its website, here is a simplified overview of the requirements for obtaining a warehouse license (third-party storage):
1. Commercial Register:
• The commercial register must include the required activity according to the approved economic activities and must be issued from the same administrative region as the facility’s location.
2. Lease Agreement:
• A signed lease agreement with the warehouse owner must exist, specifying the rented area, along with all clauses outlining the obligations of the tenant and the leasing company.
3. Required Spaces:
• The minimum rented area for third-party storage is:
• Food: 20 square meters.
• Medicines: 20 square meters.
• Cosmetics: 10 square meters.
• Medical Devices: Not specified.
4. Application for Licensing:
• The application is submitted via the Ghad platform of the SFDA, and the license is valid for one year. Upon approval from the authority, the license will be issued.
If your facility wishes to rent a partial space (third-party storage), you can check the partial space rental section. You can also explore our services and view the available prices and offers.
What are the requirements for licensing a cosmetics warehouse from the Food and Drug Authority?
There are requirements set by the Saudi Food and Drug Authority for obtaining a cosmetics warehouse license, which include:
1. Commercial Register:
The commercial register must include the specific activity for cosmetics according to the approved economic activities and must be issued from the same administrative region as the facility’s location.
2. Municipal and Civil Defense Licenses:
The warehouse must have a license from the municipality and a civil defense license to ensure compliance with safety requirements.
3. Product Transport and Distribution Agreement:
There must be an agreement for transporting products or the presence of refrigerated vehicles owned by the facility that are equipped to transport cosmetics according to the required standards.
4. Saudi Warehouse Manager:
A qualified Saudi technical manager must be registered to manage the warehouse according to the authority’s requirements.
5. Application via the Ghad Platform:
The application for obtaining a warehouse license is submitted through the Ghad platform of the Food and Drug Authority, and the license is valid for five years, with a fee of 3,000 SAR.
If your facility wishes to rent a fully equipped warehouse that meets all requirements, you can check the complete warehouse rental section (Large Enterprises Services). You can also explore the details of the licensing services we provide through the Licensing Services section.
What are the requirements for licensing a medical devices warehouse from the Food and Drug Authority?
There are requirements set by the Saudi Food and Drug Authority (SFDA) for obtaining a warehouse license for medical devices and products, which aim to ensure safe storage and compliance with health and legal standards. The requirements include:
1. Commercial Register:
The commercial register must include the activity related to the import and storage of medical devices and must be issued from the same administrative region as the warehouse location to ensure compliance with the SFDA licensing conditions.
2. Government Licenses:
The warehouse must have a municipal license and a civil defense license to ensure the safety of the building and its compliance with the standards set by the relevant government authorities.
3. Lease Agreement or Ownership Deed:
A lease agreement or ownership deed for the warehouse must be submitted as part of the warehouse licensing procedures, according to the authority’s requirements.
4. Saudi Technical Manager:
It is required to appoint a qualified Saudi technical manager registered with the SFDA to manage the warehouse and ensure compliance with health and legal standards.
5. Application via the Ghad Platform:
The application for obtaining a warehouse license is submitted through the Ghad electronic platform of the Saudi Food and Drug Authority. After submitting the application, you can track its status through the platform to ensure that all conditions are met.
6. License Duration and Fees:
The license duration is five years, and the fee for obtaining the SFDA license is 4,000 SAR. The license must be renewed before the expiration period to ensure the continuation of activities legally.
If your facility needs to rent a warehouse licensed by the SFDA for medical devices, you can visit the complete warehouse rental section within the large enterprises services. You can also check the details of the licensing services and related services in the licensing services section.
What are the conditions for importing cosmetics into Saudi Arabia?
عملية استيراد مستحضرات التجميل في السعودية تتطلب الالتزام باللوائح والإجراءات التي تضمن سلامة المنتجات وجودتها قبل دخولها السوق المحلي. تعتبر هيئة الغذاء والدواء (SFDA) الجهة المنظمة لهذا القطاع، حيث تضع شروطًا دقيقة لضمان توافق المنتجات المستوردة مع المعايير الوطنية والدولية.
شروط استيراد مستحضرات التجميل في السعودية:
تسجيل المنتج:
يجب تسجيل مستحضرات التجميل لدى هيئة الغذاء والدواء للحصول على الموافقة اللازمة.
الحصول على تراخيص الاستيراد:
يتطلب الأمر استخراج تراخيص من الجهات المختصة قبل الشروع في استيراد المنتجات.
المستندات المطلوبة:
• شهادات الجودة والمطابقة الدولية.
• تفاصيل عن مكونات المنتج و تصنيفه.
الامتثال للوائح السلامة:
جميع المنتجات المستوردة يجب أن تكون خالية من المواد المحظورة وتلتزم بمعايير السلامة والجودة.
دور شركة شركاء التطوير في تسهيل الاستيراد
نحن في شركة شركاء التطوير للخدمات اللوجستية نقدم خدمات متخصصة لدعم العملاء في جميع مراحل استيراد مستحضرات التجميل، بما في ذلك:
المساعدة في استخراج التراخيص:
• تقديم الدعم الكامل في تجهيز المستندات المطلوبة للحصول على تراخيص هيئة الغذاء والدواء.
• متابعة عملية التسجيل والتأكد من توافق المنتجات مع اللوائح.
توفير مساحات تخزين مرخصة:
• مستودعات لتخزين مستحضرات التجميل مجهزة ومرخصة من هيئة الغذاء والدواء لحفظ المنتجات بأمان.
كيفية استيراد مستحضرات التجميل بنجاح:
التعرف على الشروط واللوائح:
دراسة متطلبات هيئة الغذاء والدواء والتأكد من تلبية جميع المعايير.
التعاون مع شركاء موثوقين:
اختيار مصانع وموردين معتمدين في بلد المنشأ لضمان سير العمليات بسلاسة.
الاستعداد الكامل:
تجهيز جميع المستندات والتراخيص قبل شحن المنتجات لتجنب التأخير.
إن استيراد مستحضرات التجميل في السعودية هو عملية تتطلب دقة وخبرة لضمان الامتثال للوائح وتحقيق النجاح. مع شركة شركاء التطوير للخدمات اللوجستية، نضمن لك الدعم اللازم لتسهيل هذه العملية بكفاءة واحترافية.
تواصل معنا الآن لمعرفة المزيد عن خدماتنا وكيف يمكننا مساعدتك في كل خطوة من خطوات استيراد مستحضرات التجميل.
What are the requirements and procedure for registering a medical device with the Food and Drug Authority?
Requirements and Procedure for Registering a Medical Device with the Saudi Food and Drug Authority (SFDA):
1. Obtain a Facility License:
The facility registering the device must have an import or distribution license for medical devices issued by the SFDA, which requires a licensed warehouse license.
2. Classification of the Medical Device:
The medical device is classified based on its level of risk according to the authority’s regulations. Medical devices are typically classified into four categories based on usage risk: (Class I, Class IIa, Class IIb, Class III).
3. Registration via the Ghad Platform:
The registration request must be submitted through the Ghad electronic platform of the SFDA by creating an account and registering the facility.
4. Required Documents:
• Submit documents that include:
• Certificate of Conformity (CE or FDA) if available.
• ISO 13485 certificate proving that the company has an accredited quality management system.
• Clinical studies and technical reports demonstrating the safety and effectiveness of the device.
• A list of components and specifications of the medical device.
5. Appointment of a Local Agent:
Foreign companies must appoint a certified local agent in the Kingdom for registration and communication with the authority.
6. Payment of Fees:
Fees vary based on the category of the medical device to be registered. Fees start at 15,000 SAR and increase according to the device’s level of risk.
7. Inspection and Review:
The authority may conduct an inspection of the manufacturing or storage sites of the device to verify compliance with quality and safety requirements.
8. Follow-up After Registration:
After the registration is accepted, the facility must adhere to quality standards and maintain ongoing communication with the SFDA to ensure that data is updated if any changes occur regarding the device.
Summary Steps for Registering a Medical Device:
1. Create an account on the Ghad platform.
2. Submit the device registration request through the platform along with the required documents.
3. Wait for the authority to review the submitted documents.
4. Receive final approval upon meeting all conditions.
These are the key essential steps for registering a medical device. It is advisable to contact the Saudi Food and Drug Authority directly at 19999 or consult our specialized advisors for complete details and assistance in registering your products.
How to register or list a cosmetic product with the Saudi Food and Drug Authority via the Ghad platform?
To register or list a cosmetic product with the Saudi Food and Drug Authority, companies must adhere to several requirements and essential steps to ensure compliance with standards. The registration is done through the Ghad electronic platform, which allows the submission of the required information for cosmetic products. Here are the requirements:
1. Electronic Registration via the Ghad Platform:
• An account must be created on the Ghad platform affiliated with the Food and Drug Authority. After that, the product must be registered, and all required information must be entered.
2. Ingredients:
• The product’s ingredients must comply with the lists of permissible ingredients set by the Food and Drug Authority. The product should not contain any prohibited or harmful substances, and the list of ingredients must be clear and documented.
3. Labeling:
• A picture of the packaging and product label must be attached, including all necessary information, such as:
• List of ingredients.
• Manufacturing and expiration dates.
• Manufacturer’s name.
• Usage instructions.
• The information must be written in both Arabic and English.
4. Notification and Approval:
• After submitting the complete file, the authority reviews the documents and issues a notification of approval if the product meets the conditions. This notification allows the company to market the product in the Saudi market.
5. Fees:
• The process of registering cosmetic products is currently free, but it is always advisable to check for updated fees via the Ghad platform.
Summary Steps for Registering a Cosmetic Product:
1. Create an account on the Ghad platform.
2. Submit the product file with all required documents.
3. Wait for the authority to review the submitted file.
4. Obtain approval to start marketing the product.
To successfully complete the registration or listing process for a cosmetic product, it is advisable to contact the Saudi Food and Drug Authority for accurate and updated information or consult our specialized advisors for assistance to ensure the process is completed correctly.
























